Two Chinese medicines found to contain excessive amounts of arsenic
SINGAPORE — Retailers have been instructed to remove a batch of two Chinese Proprietary Medicines from their shelves immediately after the medicines were found to contain excessive amounts of arsenic.

SINGAPORE — Retailers have been instructed to remove a batch of two Chinese Proprietary Medicines from their shelves immediately after the medicines were found to contain excessive amounts of arsenic.
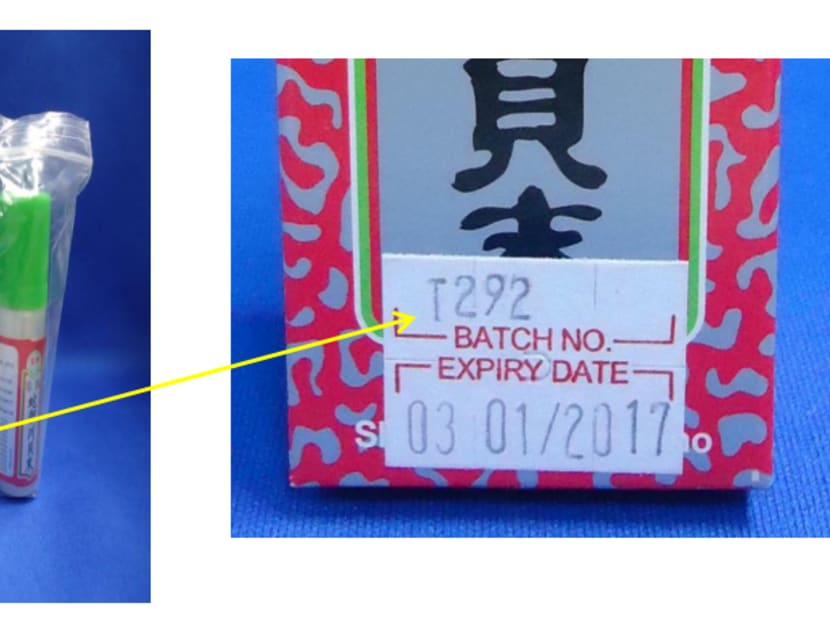
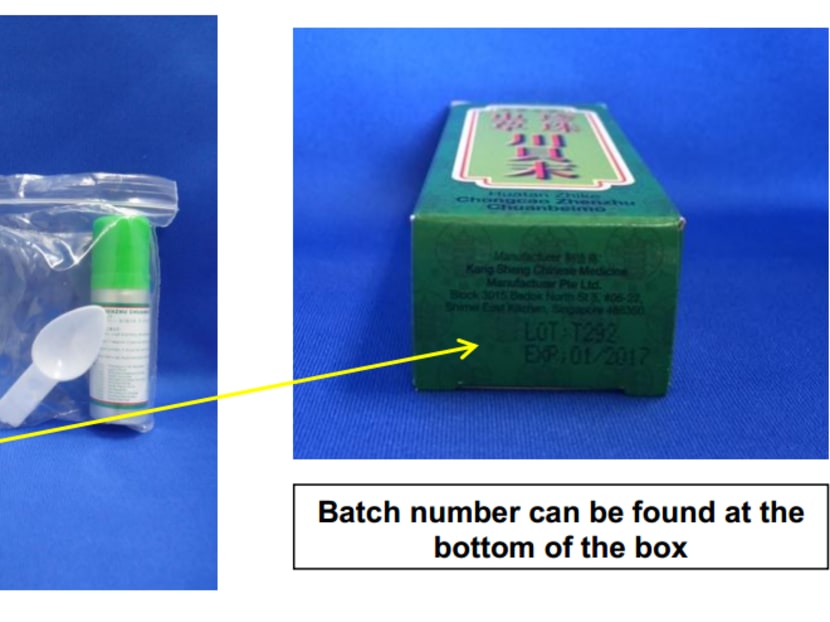
The affected batch number is T292 of the medicines, Zhenzhu Shedan Chuanbeimo and Huatan Zhike Chongcao Zhenzhu Chuanbeimo, said the Health Sciences Authority (HSA) today (Dec 18).
Existing consumers are strongly advised to stop taking the two medicines and to consult their doctors if they are feeling unwell or are concerned about their health.
They can check the batch number of their existing products through a sticker label on the packaging of Zhenzhu Shedan Chuanbeimo, or a printed number at the bottom of the box of Huatan Zhike Chongcao Zhenzhu Chuanbeimo. Consumers who are unsure of the batch they have in possession are advised not to consume the products.
Both medicines are manufactured by Kang Sheng Chinese Medicine Manufacturer and distributed locally by Tientsin Da Chong Trading. Zhenzhu Shedan Chuanbeimo is used for the treatment of high fever, cough, vomiting, phlegm and “clearing away heat and toxins”. Huatan Zhike Chongcao Zhenzhu Chuanbeimo is used for the treatment of fever, fits, cough, vomiting and phlegm.
Tientsin Da Chong Trading is currently conducting a consumer recall of the affected batch and working with the HSA to investigate this matter.
So far, there have been no reports of any consumer facing adverse reactions from the two medicines. The HSA said that possible symptoms could include vomiting, abdominal pain, diarrhoea, dark coloured urine, dehydration, heart related problems, dizziness, confusion, increased heart rate and low blood pressure.
The HSA discovered the excessive amounts of arsenic in the two medicines during a routine product quality surveillance of locally marketed health products.






