129 ‘adverse events’ reported among those aged 12 to 18; comprising 0.06% of Covid-19 vaccine doses given: HSA
SINGAPORE — As of June 30, healthcare professionals had reported 129 “adverse events” among 12 to 18-year-olds who had received the Covid-19 vaccine, comprising 0.06 per cent of the doses given to those in this age group, the Health Sciences Authority (HSA) said.
The Health Sciences Authority said on July 5, 2021 the benefits of the Pfizer-BioNTech and Moderna Covid-19 vaccines continue to outweigh the risks in the pandemic.
- 129 “adverse events” have been reported among 12 to 18-year-olds who had received the Covid-19 vaccine as of June 30
- The adverse events comprise 0.06 per cent of the doses given to those in this age group
- 12 cases of myocarditis and pericarditis were reported as of June 30, out of close to 5.5 million vaccine doses administered
- Of the 6,606 suspected adverse events, 252 were classified as serious adverse events
- The authority’s assessment is that the benefits of the vaccines continue to outweigh the known risks in a pandemic
SINGAPORE — As of June 30, healthcare professionals had reported 129 “adverse events” among 12 to 18-year-olds who had received the Covid-19 vaccine, comprising 0.06 per cent of the doses given to those in this age group, the Health Sciences Authority (HSA) said.
In an update on Monday (July 5), HSA noted that the commonly reported adverse events within this age group include rash, hives, shortness of breath, dizziness, lightheadedness and syncope, the medical term for fainting and temporary loss of consciousness.
These reported adverse events across the broader population generally resolved within a few days, and they were in line with the events reported overseas, the authority added.
The rollout of the vaccination programme for those aged 12 and above began on June 3.
All the 129 adverse events reported were associated with the use of the Pfizer-BioNTech vaccine, as it is the only vaccine approved here for those aged between 12 and 17. Those aged 18 and above can opt for either the Pfizer-BioNTech or Moderna vaccine.
Locally, 17 reports of syncope among those aged 12 to 18 years old were recorded. Most recovered after about five minutes. HSA noted that syncope is generally triggered by the vaccination process, such as anxiety about the injection and fear of pain, and not by the vaccine itself.
The local rate of syncope in this age group is about 7.4 per 100,000 doses, which is similar to the rate from overseas reports, HSA added.
This is HSA’s third safety update on the vaccines used in Singapore, with the previous update published last month.
RARE CASES OF MYOCARDITIS AND PERICARDITIS
In its report, HSA also noted 12 cases of myocarditis and pericarditis across the broader population as of June 30, out of close to 5.5 million vaccine doses administered.
Myocarditis refers to the inflammation of the heart muscles, while pericarditis refers to inflammation of the outer lining of the heart. Typical symptoms are chest pain, shortness of breath and a fast heartbeat.
HSA said that the inflammation was mild in most cases.
Five of the cases occurred in adults aged 30 and above, comprising three men and two women, which was within the baseline incidence rate.
Seven of the cases involved males aged below 30 and this exceeds the expected numbers for this age group, based on background incidence rates.
While most of the cases reported previously occurred after the second dose, HSA said that it also received reports of six cases that occurred after the first dose. Most of the cases were reported to have occurred within a week after vaccination.
“Although there is a small increased risk of myocarditis and pericarditis, the local incidence rate remains low. The overall local incidence rate is 0.22 per 100,000 doses administered, and the incidence rate in males below 30 years old is 1.24 per 100,000 doses administered,” HSA said.
“All the cases in the younger age group below 30 years old responded well to treatment and had recovered or were discharged well from the hospital.”
More broadly, as of June 30, 6,606 suspected adverse events had been reported out of a total of 5,470,425 vaccine doses given, comprising 0.12 per cent of administered doses.
HSA added that most of the adverse events reported were associated with the Pfizer-BioNTech vaccine, as it comprised 79 per cent of the doses administered in the population, as it was rolled out on Dec 30, 2020, while Moderna was administered from March 12 this year.
COMMONLY REPORTED ADVERSE EVENTS
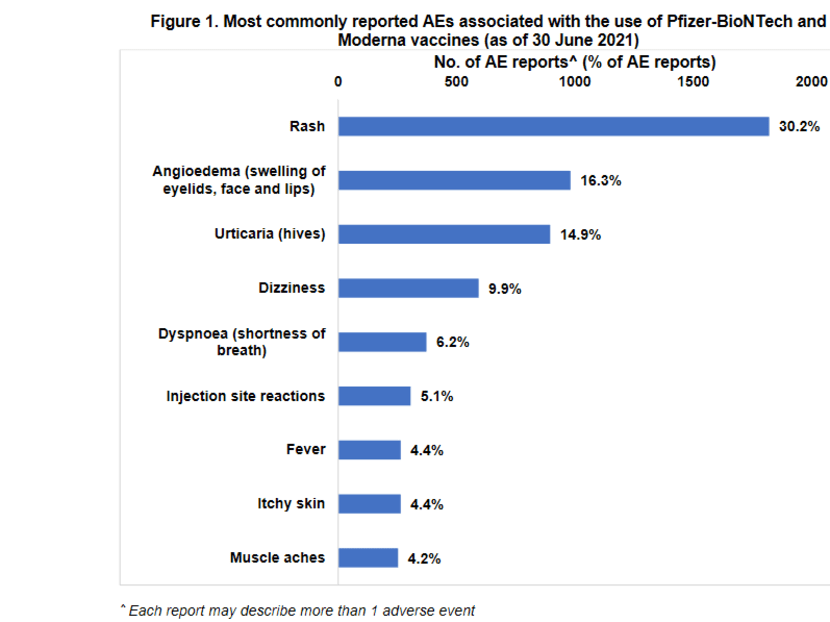
HSA noted that the most commonly reported adverse events were consistent with those typically observed following vaccination.
These include injection site reactions such as pain and swelling, dizziness, fever, muscle aches, shortness of breath and allergic reactions such as rash, itch, hives and swelling of eyelids, face and lips.
These adverse events generally resolved within a few days, and “are also in line with those reported overseas”.
SERIOUS ADVERSE EVENTS
Of the 6,606 suspected adverse events, 252, comprising 0.005 per cent of doses administered, were classified as “serious” adverse events.
Adverse events are classified as serious or severe if they result in hospitalisation, disability or a life-threatening illness, among other reasons.
The authority noted that the most frequently reported serious adverse events were anaphylaxis (42 reports) and other severe allergic reactions (32 reports).
Other serious adverse events include exacerbation of underlying asthma condition, breathing difficulty, fast heart rate, an increase or decrease in blood pressure, chest discomfort and pain, pericarditis or myocarditis, syncope, limb numbness, weakness or pain, changes in vision, increase in liver enzymes, joint pain, seizures (fits), tinnitus (ringing in the ears) and infections.
“These serious adverse events are being closely monitored by HSA,” the authority said. It added that most of the individuals who developed serious adverse events were reported to have recovered or were recovering.
“No deaths suspected to be associated with the vaccines or any other causes have been reported locally.”
ADVERSE EVENTS OF SPECIAL INTEREST
HSA also said that anaphylaxis and Bell’s Palsy are examples of adverse events of special interest, which is a “pre-specified medically significant event that has been observed historically with other vaccines”.
There were 42 cases of anaphylaxis reported with the Pfizer-BioNTech and Moderna vaccines, and all the patients were reported to have recovered after medical treatment.
Anaphylaxis refers to a rare and potentially life-threatening allergic reaction that can occur following vaccination in certain susceptible individuals.
HSA said that the incidence rate of anaphylaxis reported in Singapore is about 0.81 per 100,000 doses administered.
This is similar to the incidence rates reported overseas of around 0.5 to 2 per 100,000 doses administered.
In addition, 62 cases of Bell’s Palsy have been reported, with most of the reports being non-serious. It is a condition that causes temporary weakness or paralysis of the facial muscles.
Most of the patients with Bell’s Palsy will generally recover completely even without treatment, HSA said.
The local incidence rate is estimated to be 2.3 per 100,000 persons per month, which is within the background incidence rate of 1.1 to 4.4 per 100,000 persons per month prior to the introduction of vaccination.
The authority also noted that a greater frequency of heart attacks and strokes has not been observed in vaccinated persons locally.
HSA said that “most of the adverse events are largely expected with vaccination and reflect what has been reported in the clinical trials”.
It added that its “current assessment is that the benefits of the Pfizer-BioNTech and Moderna vaccines continue to outweigh the known risks in a pandemic”.
Source: Health Sciences Authority









