2 suffer adverse effects after taking health products with potent ingredients, banned substance: HSA

Traditional Herbs Preparation XPE (left) and FS++ Slimming Supplements By JPJ Slim (right) were found to contain ingredients that can cause serious effects.
SINGAPORE — The Health Sciences Authority (HSA) on Tuesday (March 1) warned the public not to consume two health products found to have "potent adulterants", after two people reported adverse effects from taking them.
SINGAPORE — The Health Sciences Authority (HSA) on Tuesday (March 1) warned the public not to consume two health products found to have "potent adulterants", after two people reported adverse effects from taking them.
The two products are:
- Traditional Herbs Preparation XPE
- FS++ Slimming Supplements By JPJ Slim
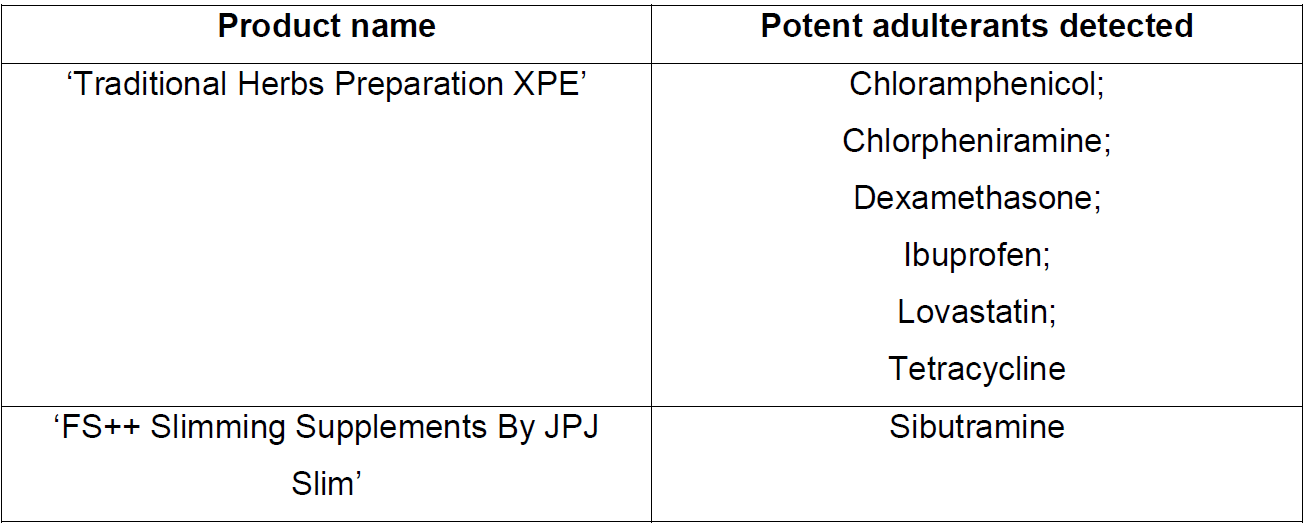
Six potent medicinal ingredients including steroids and antibiotics were found in the first product, while the second contained the banned substance sibutramine, HSA said in a media release.
“These adulterants can cause serious adverse effects in individuals,” it added.
JOINT PAIN WORSENED
A woman in her 60s had been taking "Traditional Herbs Preparation XPE" for over nine months to alleviate her joint pain. She had received it from a friend who obtained it in Malaysia, HSA said.
While she experienced a quick relief of her joint pain, it worsened when she stopped taking it or reduced the dose, leading her doctor to suspect that the product may contain undeclared ingredients such as steroids or painkillers.
HSA tested the product and found six medicinal ingredients:
- dexamethasone (a steroid)
- chlorpheniramine (an antihistamine)
- ibuprofen (a non-steroidal anti-inflammatory drug)
- lovastatin (a cholesterol-lowering medicine)
- chloramphenicol and tetracycline (both antibiotics)
Long-term unsupervised use of steroids such as dexamethasone can cause increased blood glucose levels leading to diabetes, Cushing’s syndrome — characterised by a round or "moon face" appearance, and upper body central obesity with thin limbs — as well as other serious side effects.
INSOMNIA, CONFUSION
Another consumer experienced insomnia, headache and confusion after taking "FS++ Slimming Supplements" that was bought from a social media platform here, which carried consumer testimonials on the product's quick slimming results.
It was found to have sibutramine, a prescription-only weight-loss medicine that was banned in 2010 because of an increased risk of heart attack and strokes.
Continued use could lead to serious health consequences such as heart problems, psychosis and hallucinations, HSA said.
HSA added that it has since worked with the platform administrators to promptly remove the affected listings. Investigations against the seller are ongoing.
Consumers who have bought or taken any of the products are advised to stop using them immediately and see a doctor if they are unwell or concerned about their health.
Sellers and suppliers of these products must also stop selling them immediately.
Those convicted of selling and supplying products found to be adulterated with potent medicinal ingredients can be jailed for up to two years or fined up to S$10,000, or both.
HSA advised the public to be wary of products that carry exaggerated claims of delivering unexpectedly quick results, because they may contain ingredients that can cause serious harm to one’s health.
Consumers should also exercise caution when buying products online or from friends.
The authority added that there was no “quick and easy” way to lose weight and that people should control their weight through a balanced diet and exercise.











