Explainer: What are the alternate Covid-19 vaccines, how do these differ from Pfizer-BioNTech and Moderna?
SINGAPORE — The recent decision by the Government to relax Covid-19 vaccination rules will soon allow Singapore residents more access to alternate vaccines, with the first among them likely to be China’s Sinovac.
- Non-mRNA Covid-19 vaccines may be suitable for individuals who are unable to get inoculated with Pfizer-BioNTech and Moderna
- However, they still come with risks of side effects
- Infectious disease experts said individuals should still consult their doctors being getting inoculated
- Alternate vaccines are not part of the national vaccine programme
- This means they are not free and individuals will not be protected by a government financial aid scheme
SINGAPORE — The recent decision by the Government to relax Covid-19 vaccination rules will soon allow Singapore residents more access to alternate vaccines, with the first among them likely to be China’s Sinovac.
The Ministry of Health (MOH) said on Monday (May 31) that it will allow a special route for private healthcare providers to administer Covid-19 vaccines that are on the Emergency Use Listing (EUL) of the World Health Organization (WHO).
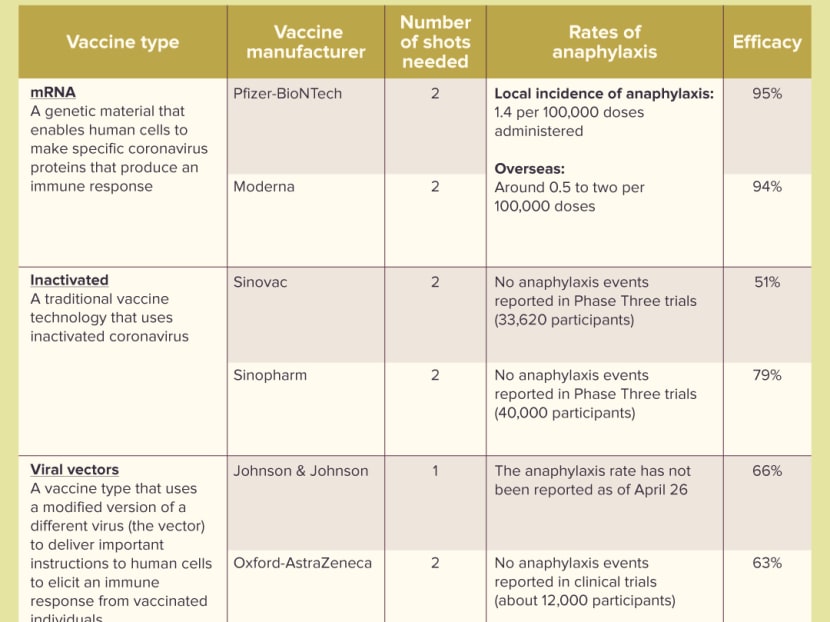
The list comprises the Pfizer-BioNTech and Moderna vaccines that uses messenger ribonucleic acid (mRNA) technology, as well as viral vector vaccines such as Oxford-AstraZeneca and Johnson & Johnson, and inactivated vaccines Sinopharm and Sinovac.
MOH said in response to media queries on Wednesday that it will release more details in the coming days for private healthcare institutions to apply to be licensed providers for the Sinovac vaccine.
It is also studying the possibility for private healthcare institutions to access the 200,000 Sinovac doses in stock, and work out details on pricing, informed consent process and the safety of the patients who prefer to be administered with it.
Some private healthcare groups and general practitioners told TODAY that they are awaiting more guidelines from MOH before they make a decision on providing the alternate vaccines on WHO's list.
Farrer Park Hospital said that it is working with the Health Sciences Authority (HSA) to secure Sinovac's Covid-19 vaccine, though the pricing and number of doses have yet to be ascertained at this point.
It added that both the doctor administering the vaccine and the patient would have to discuss the risks and benefits of using vaccines not registered or authorised by HSA. Before the vaccination, patients will also have to sign an informed consent form to acknowledge the risks involved.
Health Minister Ong Ye Kung said on Monday that there are more than 30,000 individuals in Singapore who are unable to take the mRNA-based vaccines for medical reasons.
He noted that some want alternate vaccines because of their history of anaphylaxis — a severe, life-threatening allergic reaction — which restricts them from taking mRNA vaccines.
The mRNA vaccines, when injected, "instruct" cells in the human body to make a protein similar to a fragment of a virus, so that the body produces antibodies and special immune system cells in response.
Some infectious disease experts told TODAY that while non-mRNA vaccines may not be as effective, they have been reported to have a lower rate of severe allergic reactions.
TODAY takes a closer look at the four non-mRNA vaccines on the EUL, including costs, availability and how they differ from the Pfizer-BioNTech and Moderna vaccines.
WHAT IS THE EUL?
WHO defines it as a procedure for assessing unlicensed vaccines, therapeutics and in-vitro diagnostics during public health emergencies, with the ultimate goal of speeding up the availability of these products to people who need them.
WHAT IS THE DIFFERENCE BETWEEN THE VACCINES?
Both Pfizer-BioNTech and Moderna make use of mRNA technology.
Pfizer-BioNTech has an efficacy rate of 95 per cent, while for Moderna, it is 94 per cent. Both are offered for free to all Singaporeans and long-term residents here.
Here is how they compare to other vaccines on the EUL.
Inactivated vaccines: This is traditional vaccine technology that uses inactivated coronavirus to trigger an immune response. This is a similar method vaccines for diseases such as polio use.
Sinopharm has an efficacy rate of 79 per cent, while Sinovac’s is 51 per cent.
Viral vectors: This vaccine type uses a modified version of a different virus (the vector) to deliver important instructions to human cells to elicit an immune response from vaccinated individuals.
Oxford-AstraZeneca has an efficacy rate of 63 per cent, while Johnson & Johnson’s is 66 per cent.
ARE NON-MRNA VACCINES SAFER?
Professor Dale Fisher, a senior infectious disease consultant at the National University Hospital, said that mRNA vaccines have “come through as a stellar platform” during the pandemic due to their high efficacy rate.
That said, infectious disease specialist Leong Hoe Nam noted that Singapore was previously adopting a “one-size-fits-all for everyone” by only allowing the use of Pfizer-BioNTech and Moderna vaccines.
“But it doesn't work that way,” he said. “For some individuals, you need an alternative vaccine, such as those with multiple allergies.”
Professor Paul Tambyah, president of the Asia Pacific Society of Clinical Microbiology and Infection, said that the rates of anaphylaxis reported with the viral vector vaccines and the inactivated vaccines are reportedly lower than with the mRNA vaccines.
In an April 18 safety update by HSA, it was stated that the incidence rate of anaphylaxis reported here with Pfizer-BioNTech and Moderna vaccines is about 1.4 for every 100,000 doses administered.
This is similar to the incidence rates reported overseas of around 0.5 to 2 for every 100,000 doses administered.
In comparison, a report published by the World Allergy Organization in January this year stated that no anaphylaxis events have been reported in clinical trials for Sinovac, Sinopharm and Oxford-AstraZeneca.
The American College of Allergy, Asthma and Immunology said that the anaphylaxis rate has not been reported for the Johnson & Johnson vaccine as of April 26.
In any case, Prof Tambyah said that the viral vector vaccines come with their own risks of side effects such as rare blood clots, so individuals need to talk to their doctors about the risks and the benefits of the different vaccines.
WHAT HAPPENS IF YOU FALL ILL TAKING AN ALTERNATIVE VACCINE?
In short, you will not be protected.
MOH said that the vaccines are not part of the national vaccine programme, therefore individuals will not be eligible for the Vaccine Injury Financial Assistance Programme for Covid-19 Vaccination should they fall ill after getting inoculated.
The scheme provides one-time goodwill financial assistance to persons who experience serious side effects that are assessed to be related to Covid-19 vaccines administered in Singapore.
WHAT IS THE PRODUCTION CAPACITY OF ALTERNATE VACCINES?
Sinovac: In early April, media reports stated that the Chinese biopharmaceutical firm would be doubling its annual capacity of the jabs to two billion doses.
The firm said that it has supplied more than 600 million doses at home and abroad as of the end of May.
Sinopharm: Reuters reported on Tuesday that the firm was raising its annual production capacity of its Covid-19 vaccine to at least one billion doses.
The news agency reported in March that more than 100 million doses of Sinopharm’s vaccine have been supplied around the world.
Oxford-AstraZeneca: The company AstraZeneca said that its goal was to produce up to three billion doses of its vaccine by this year.
It said in May that it has supplied more than 400 million doses worldwide.
Johnson & Johnson: The company said in March that it intends to deliver one billion doses of its vaccine by the end of the year.
While there are no updated reports of how many doses have been produced so far, the New York Times reported in late March that up to 15 million doses of the vaccine had been ruined due to human error.
Dr Leong said that one concern he has about the procurement process of the vaccines is whether medical practitioners are able to procure a genuine version of the vaccine.
Although MOH did not respond to TODAY’s queries about the procurement process, a general practitioner who declined to be named said that there will typically be a list of HSA-approved vendors when it comes to the purchasing of any vaccine.
HOW MUCH ARE OTHER COUNTRIES PAYING A DOSE?
Prof Tambyah said that the one key issue about alternate vaccines in Singapore will be the cost, since it will not be subsidised by the Government.
“This raises questions about inequity but unfortunately, that is the nature of healthcare systems such as ours or that of the United States.”
The price for the various alternate vaccines varies not only according to type, but country to country as well.
For instance, the peer-reviewed medical trade journal The BMJ said in a Jan 29 publication that in South Africa, the Oxford-AstraZeneca vaccine costs around US$5.25 (S$7) a dose, more than double what the European Union (EU) was paying at US$2.15.
The EU is also paying US$8.50 for the single-dose Johnson & Johnson vaccine as well.
In a separate article in April, the journal said that the cost of each Sinopharm and Sinovac dose in China was around US$30.
Whatever the ultimate cost will be in Singapore, Prof Tambyah said that having an alternate vaccine source for residents here is a good move as it provides more options.
“The government ministers mentioned vaccine supply as a limiting factor in pandemic control, so now the gate has been opened,” he said.
“I am sure that many providers will rise to the challenge and we will be able to vaccinate even more people and move closer to opening up.”